This week we celebrate pineapples, in honor of the completion of the sequencing of the pineapple (Ananas comosus) genome and the insights it provides into an important metabolic pathway (Ming et al., 2015). Pineapples are more than just tasty tropical fruit, they’re also one of many plants able to carry out a special form of photosynthesis known as CAM; other CAM plants include various species within the genus Agave (including Agave tequilana, the source of the drink tequila), Kalanchoe species, Mesembryanthemum crystallinum (often called ice plant) Opuntia ficus-indica (prickly pear cactus) and Sedum species (stonecrops). CAM confers upon these plants the ability to thrive in very dry environments, and it has evolved repeatedly in over 35 plant families. The water-efficiency of CAM plants makes them very attractive candidates for biofuel and food production in water-limited areas (Borland et al., 2014; Cushman et al., 2015; Yang et al., 2015).
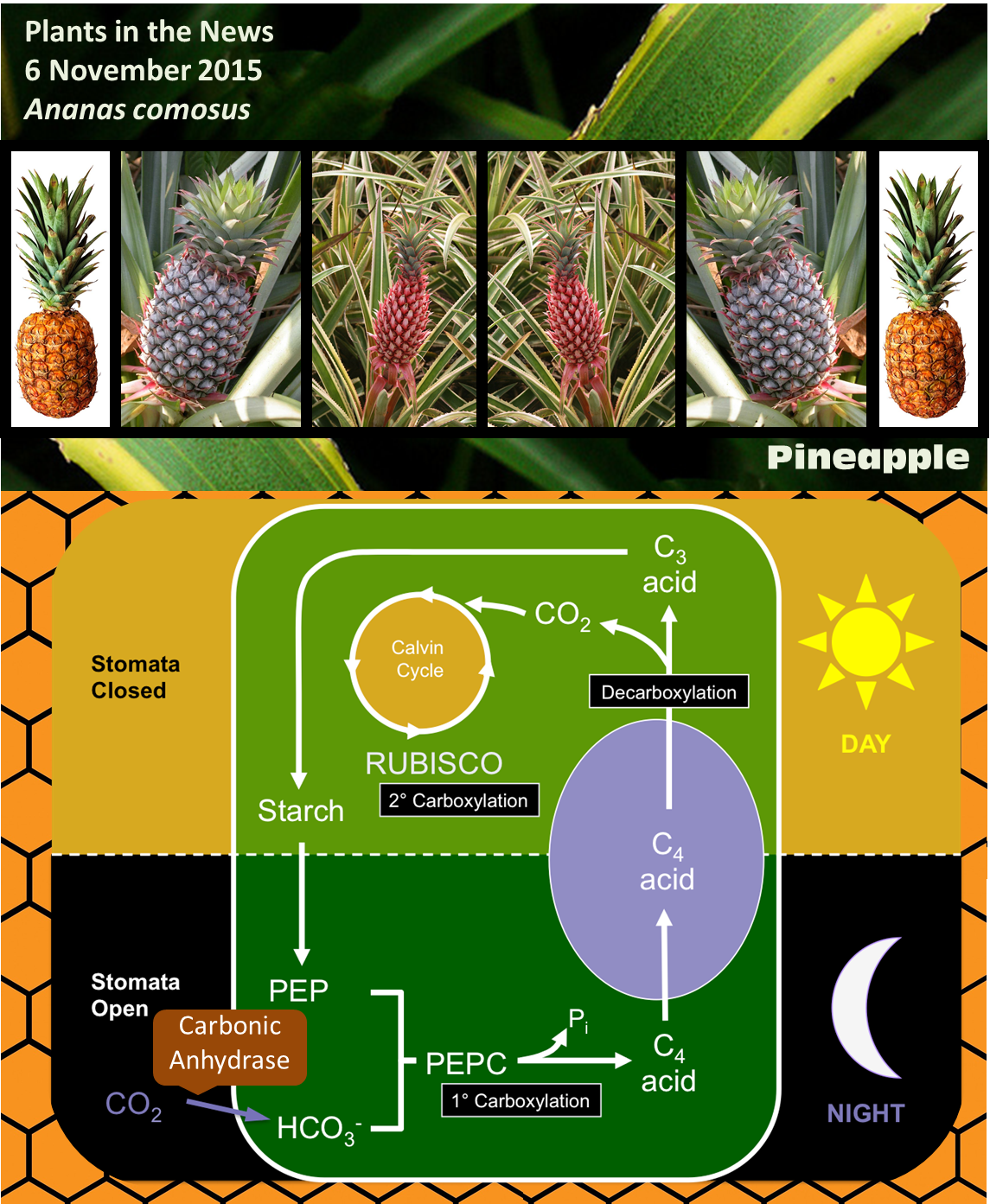
CAM stands for Crassulacean acid metabolism. CAM photosynthesis is a variation of the normal carbon fixing pathway, and was first discovered in members of the Crassulacea family. Most plants use a type of carbon fixation known as C3 photosynthesis, in which the enzyme RUBISCO (ribulose bisphosphate carboxylase/oxygenase) adds a molecule of CO2 to a five-carbon sugar (ribulose bisphosphate) to form a six-carbon compound. However, this six-carbon compound is quite unstable and quickly splits into two 3-carbon compounds, thus the name C3.
Carbon dioxide enters plants through pores called stomata, but when these pores are open the plants also lose water through the process of transpiration. RUBISCO requires high concentrations of CO2 to work effectively, and carries out a competing oxidation reaction when CO2 levels are low. Two similar metabolic variants that increase the efficiency of carbon fixation have evolved several times in plants in dry environments; these variations allow the plants to lose less water but still maintain a high rate of CO2 fixation, and both involve another enzyme called PEPC [phosphoenolpyruvate (PEP) carboxylase].
In C4 plants, PEPC attaches CO2 to a three-carbon compound (PEP) to produce a four-carbon compound; thus the name C4. The four-compound is decarboxylated in another cell that contains RUBISCO, essentially concentrating CO2 and lowering the rate at which RUBISCO carries out the competing oxygenation reaction.
In CAM, like C4, CO2 is first fixed by PEPC. The difference is that CAM plants take up CO2 and fix it at night, when temperatures are lower and the rate of transpiration is much lower than during the day. It is often said that C4 plants separate CO2 fixation and RUBISCO activity spatially, whereas CAM plants separate them temporally (Cushman and Bohnert, 1990). Interestingly, some plants are facultative CAM plants, meaning that they can shift to CAM for example under drought stress (Winter and Holtum, 2014).
Some of the genes associated with CAM, including those encoding the key enzyme carbonic anhydrase (which converts CO2 to bicarbonate, which is required for PEPC to do its job) show the expected patterns of circadian expression (on at night and off during the day). One key finding of the new paper by Ming et al is that some of their promoters have binding sites for transcriptional regulators already identified as controlling circadian patterns of expression. Although circadian activity cycles for CAM enzymes have been shown previously, this is the first time a direct cis-regulatory mechanistic link has been made between circadian regulation and CAM gene expression.
This study takes us one step closer to the possibility of transferring the trait of fixing carbon using CAM to other plants, which could confer to other plants this water-conserving capability. The fact that CAM has evolved repeatedly suggests that this is feasible, and given that CAM occurs in only a single cell rather than being distributed across two cells means that it might be easier than engineering C4 photosynthesis (Davis et al., 2014; DePaoli et al., 2014). The pineapple genome also provides insights into the regulation and evolution of CAM, and will provide invaluable in the breeding of CAM plants for bioenergy production (Borland et al., 2014; Cushman et al., 2015; Davis et al., 2014; Yang et al., 2015).
References
Borland, A.M., Hartwell, J., Weston, D.J., Schlauch, K.A., Tschaplinski, T.J., Tuskan, G.A., Yang, X. and Cushman, J.C. (2014). Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci. 19: 327-338.
Cushman, J.C. and Bohnert, H.J. (1999). Crassulacean acid metabolism: Molecular Genetics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 305-332.
Cushman, J.C., Davis, S.C., Yang, X. and Borland, A.M. (2015). Development and use of bioenergy feedstocks for semi-arid and arid lands. J. Exp. Bot. 66: 4177-4193.
Davis, S.C., LeBauer, D.S. and Long, S.P. (2014). Light to liquid fuel: theoretical and realized energy conversion efficiency of plants using Crassulacean Acid Metabolism (CAM) in arid conditions. J. Exp. Bot. 65: 3471-3478.
DePaoli, H.C., Borland, A.M., Tuskan, G.A., Cushman, J.C. and Yang, X. (2014). Synthetic biology as it relates to CAM photosynthesis: challenges and opportunities. J. Exp. Bot. 65: 3381-3393.
Ming, R., et al.,(2015). The pineapple genome and the evolution of CAM photosynthesis. Nat Genet. advance online publication
Winter, K. and Holtum, J.A.M. (2014). Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 65: 3425-3441.
Yang, X., et al., (2015). A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol. 207: 491-504.
Image credits: CAMBioDesign, Alvesgaspar,

Hi Mary Williams , that was an interesting read ! Do you have a linked in account ? So that I can message you ?
You can find my email address on the ASPB website if you want to get in touch with me,
-Mary
Engineering CAM pathways into C3 crops has a great possibility to enhance the water use efficiency of crops but the inverse stomatal regulation mechanism, if introduced successfully in C3, will keep the stomata closed during the daytime. It will create a barrier to the entry of CO2 from the atmosphere into the leaf mesophyll cells until sun sets and stomata opens. C4 plants keep stomata open during daytime. where the carboxylation efficiency will possibly be more then; if C3 converted into C4 or CAM plants ?
Have a look at the article by Borland et al (Trends in Plant Science 2014). Their article addresses all of the changes that would have to be made to introduce CAM to C3 plants, including the ability to store carbon overnight. Converting C3 plants to C4 is another good possibility and would have the same water-use efficiency benefits – the C4 rice project has a lot of information about this strategy http://c4rice.irri.org/. I think it’s good to pursue both possibilities.