Learning to read a scientific paper is an important skill for undergraduate students to acquire, but selecting a suitable paper to read with undergraduates can be challenging (see this for example). The chosen research article should be accessible (meaning not too much specialized terminology or methodology), interesting, and meaningful.
A new Plant Physiology paper by Cui et al, “Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant, Phtheirospermum japonicum” satisfies all three criteria. The terminology and methods are accessible, and the topic of how parasitic plants adhere to their hosts is interesting and meaningful.
A particular appeal of this paper is that parasitic plants are both inherently interesting and significant causes of crop losses (Scholes and Press, 2008; Spallek et al., 2013; Westwood et al., 2010). Parasitism has arisen independently multiple times, and about 1% of angiosperms have evolved the ability to parasitize other plants. Parasitism requires the ability to recognize and respond to the host as well as the aiblity to form specialized tissues for host invasion. Parasitic plants are more than just botanical curiosities though; in some tropical regions, such as the savanna agriculture in Africa, they are major agricultural pests and can impact the food security of more than 100 million people.
Parasitic plants invade host tissues by forming specialized structures called haustoria (Westwood et al., 2010; Yoshida and Shirasu, 2012). Diverse signals from the host called haustorium-inducing factors contribute to haustoria formation (note that these are distinct from the strigolactones that promote seed germination). The identification of 2,6-dimethoxy-p-benzoquinone (DMBQ) as a haustrorium-inducing factor provides a straightforward design for a genetic screen. In this study, the authors focus on mutants that are defective in the production of haustorial hairs.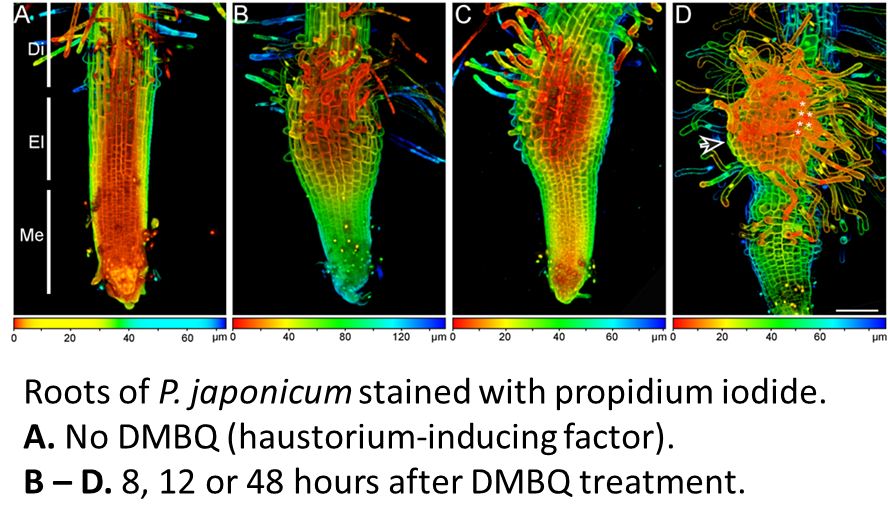
Using the facultative parasitic plant Phtheirospermum japonicum, Cui et al identified mutants with defects in the formation of haustorial hairs (hhd1 and hhd2), to investigate the developmental origin and function of these hairs. The data provided in Tables 1 and 2 provide a nice opportunity for students to interpret genetic data from crosses between the mutants and wildtype (to determine whether the alleles are dominant or recessive) and between mutants (to determine complementation groups).
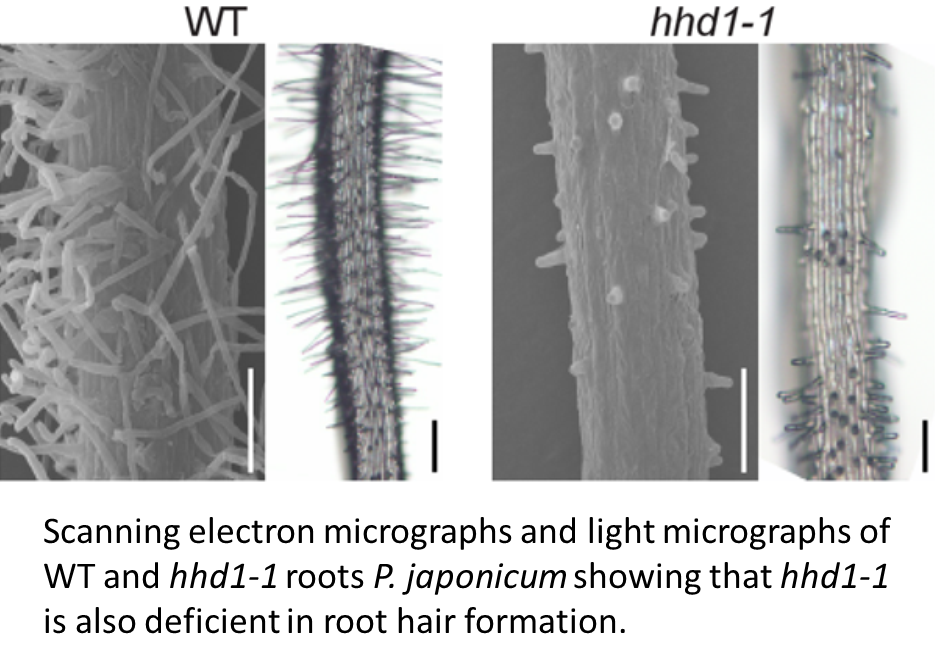
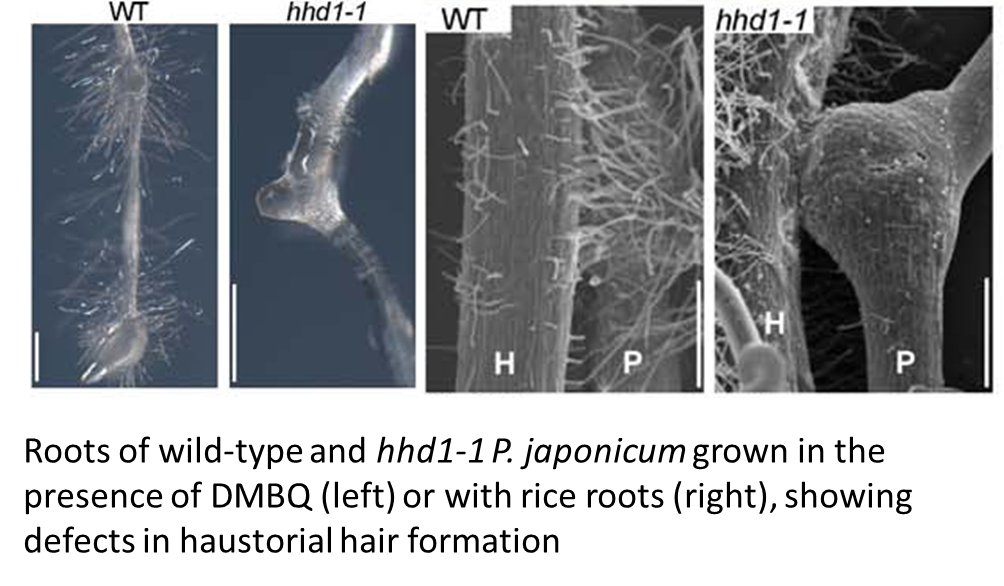 The authors investigate the developmental origin of the haustorial hairs by observing that hhd1 and hhd2 show deficiencies in both haustorial hair formation and root hair formation, and that the haustorial hairs express a root-hair specific marker. Conversely, hhd1 and hhd2 show no abnormality in the development of specialized intrusive cells, which are thought to have a distinct function. These studies provide a nice example of how phenotypic characterization of a mutant provides insights into developmental processes, and also use a variety of common imaging methods.
The authors investigate the developmental origin of the haustorial hairs by observing that hhd1 and hhd2 show deficiencies in both haustorial hair formation and root hair formation, and that the haustorial hairs express a root-hair specific marker. Conversely, hhd1 and hhd2 show no abnormality in the development of specialized intrusive cells, which are thought to have a distinct function. These studies provide a nice example of how phenotypic characterization of a mutant provides insights into developmental processes, and also use a variety of common imaging methods.
 Finally, the authors examine the mutants’ response to proximity to or close contact with roots of the host plants. Interestingly, in the hhd1 and hhd2 mutants, fewer haustoria are formed in proximity to the host, but when forced into close contact haustoria formation is restored. The authors suggest that the haustorial hairs ensure close contact between parasite and host root, which is necessary for efficient parasitism.
Finally, the authors examine the mutants’ response to proximity to or close contact with roots of the host plants. Interestingly, in the hhd1 and hhd2 mutants, fewer haustoria are formed in proximity to the host, but when forced into close contact haustoria formation is restored. The authors suggest that the haustorial hairs ensure close contact between parasite and host root, which is necessary for efficient parasitism.
After reading this article, students should be able to suggest additional studies for further research. These could include studies to identify the gene products of HHD1 and HHD2, and to examine the efficiency of parasitism of these mutants in field conditions. Students could also be asked to speculate on how this research could be used to suppress rice parasitism by P. japonicum and other parasites; examples could include efforts to block haustorial hair formation in the parasites or attachment sites in the host, or to affect the production or perception of haustrorium-inducing factors. Reading this paper could also inspire students to explore related topics including strategies to mitigate the effects of parasitic plants, the role of strigolactones in host-parasite interactions, the evolution of parasitism, root hair development, and mechanisms of plant resistance to pathogens.
Finally, if your students are unfamiliar with reading the scientific literature, we have produced a guide, “How to read a scientific paper” to help students learn this important skill.
Do you have a favorite paper you recommend for reading with undergraduates? Share below or email mwilliams@aspb.org if you’d like to write about it here.
References
Cui, S., Wakatake, T., Hashimoto, K., Saucet, S., Toyooka, K., Yoshida, S. and Shirasu, K. (2015). Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant, Phtheirospermum japonicum. Plant Physiology (in press). doi:10.1104/pp.15.01786.
Scholes, J.D. and Press, M.C. (2008). Striga infestation of cereal crops – an unsolved problem in resource limited agriculture. Curr. Opin. Plant Biol. 11: 180-186.
Spallek, T., Mutuku, M. and Shirasu, K. (2013). The genus Striga: a witch profile. Molecular Plant Pathology. 14: 861-869.
Westwood, J.H., Yoder, J.I., Timko, M.P. and dePamphilis, C.W. (2010). The evolution of parasitism in plants. Trends Plant Sci. 15: 227-235.
Yoshida, S. and Shirasu, K. (2012). Plants that attack plants: molecular elucidation of plant parasitism. Curre. Opin. Plant Biol. 15: 708-713.
